|
Multikine® Phase III Clinical Trial Design and Status
The end game is near: The final stages of clinical trials: Multikine® has been approved for a global Phase III trial. It has received a go-ahead by the US FDA as well as the Canadian regulators. This trial is expected to be the largest head and neck cancer clinical study ever conducted.
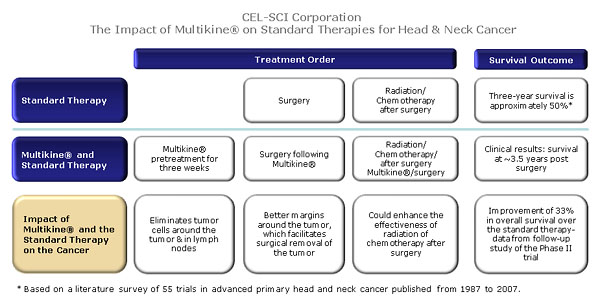
Because of Multikine’s orphan drug status, only one pivotal Phase III trial is needed for Multikine’s approval. Advanced primary (not yet treated) head and neck cancer represents an unmet medical need (about 50% of the patients die within three years following treatment) with minimal progress over the last 50 years.
The pivotal Phase III study will enroll approximately 880 subjects (in order to have approximately 784 evaluable patients) with advanced primary head and neck cancer worldwide. The Phase III open-label, randomized, controlled, multi-center study will be conducted in about 48 centers in 9 countries and in 3 continents.
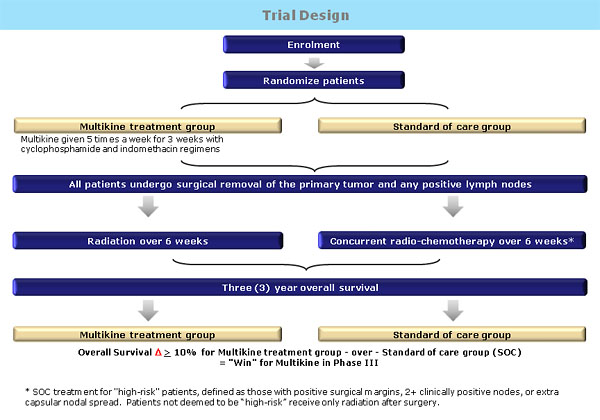
The trial is designed to compare Multikine® combined with the current standard of care therapy (SOC) for head and neck cancer patients vs. the SOC for these patients alone. SOC therapy is the therapy that patients are expected to receive as a matter of routine. The current SOC for head and neck cancer is surgery, followed by radiation, or surgery followed by radiation and concurrent chemotherapy.
If Multikine® proves to be beneficial to patients in the Phase III trial, the study will have established a new SOC for newly diagnosed head and neck cancer patients of which Multikine® will be an integral part. This means that every newly diagnosed head and neck cancer patient with advanced primary disease would be expected to receive Multikine®, and reimbursement would be customary.
This study is also the first Phase III study in the world in which immunotherapy is given to patients first, i.e. prior to receiving any conventional treatment for cancer, including surgery, radiation and/or chemotherapy. This is significant because conventional therapy weakens the immune system, and likely compromises the benefits of immunotherapy. Multikine works by activating the intact immune system since it is given before the ravages of conventional cancer therapy.
Study Summary: A Pivotal Phase III, Open-label, Randomized, Multi-center Global Study of the Effects of Multikine Plus Standard of Care (Surgery + Radiotherapy or Surgery + Concurrent Chemoradiotherapy) in Subjects with Advanced Primary Squamous Cell Carcinoma of the Oral Cavity Versus Standard of Care Only.
Objectives: The primary objective is to determine the efficacy of peri-tumoral and peri-lymphatic injection of Multikine given prior to Standard of Care (SOC) as measured by overall survival. The secondary objectives are to evaluate the effects of Multikine® treatment on the cumulative incidence of local-regional control, progression-free survival, tumor response, tumor histopathology, and quality of life, while confirming Multikine® safety.
Number of Subjects: 880
Clinical Centers: approximately 48 globally distributed; North America, Europe, Asia)
Statistics: 80% Power; 95% confidence - to show a 10% Overall Survival advantage
Steps taken to reduce the risk of failure of the Multikine Phase III trial:
The most common reasons for Phase III study failures or the failure to receive approval to sell a drug, other than the drug not working, are:
| 1. |
Phase III study that is not reviewed by the FDA and not acceptable to the FDA:
Multikine Phase III study was reviewed in detail and changes were made to the Phase III protocol based on FDA’s comments.
|
| |
|
| 2. |
A study that is too small:
The Multikine study will enroll 880 patients, which is a very large number. |
| |
|
| 3. |
The clinical endpoints are not relevant:
The Multikine study follows the overall survival of the patients, which is the gold standard. The clinical endpoint cannot be more relevant. |
| |
|
| 4. |
A change in treatment protocol between Phase II and Phase III without additional studies:
The Multikine Phase III study is the same as the Phase II study; no changes were made. Consequently, we expect the Phase II results to be representative of the results one can expect in the Phase III trial. |
| |
|
| 5. |
Insufficient attention to manufacturing issues:
The Multikine manufacturing process is validated and CEL-SCI has built a dedicated manufacturing facility for Multikine. The Manufacturing Facility is designed and built to meet US FDA and EU regulations and requirements for the manufacture of Sterile injectable products (such as Multikine). |
The general schema and comparator arms of the Phase III trial are depicted in the Trial design cartoon (below, click image to enlarge).
|