|
CEL-SCI CORPORATION REPORTS THIRD QUARTER 2010 FINANCIAL RESULTS
Vienna, VA, August 16, 2010 -- CEL-SCI Corporation (NYSE AMEX: CVM) reported today financial results for its third fiscal quarter for the period ended June 30, 2010.
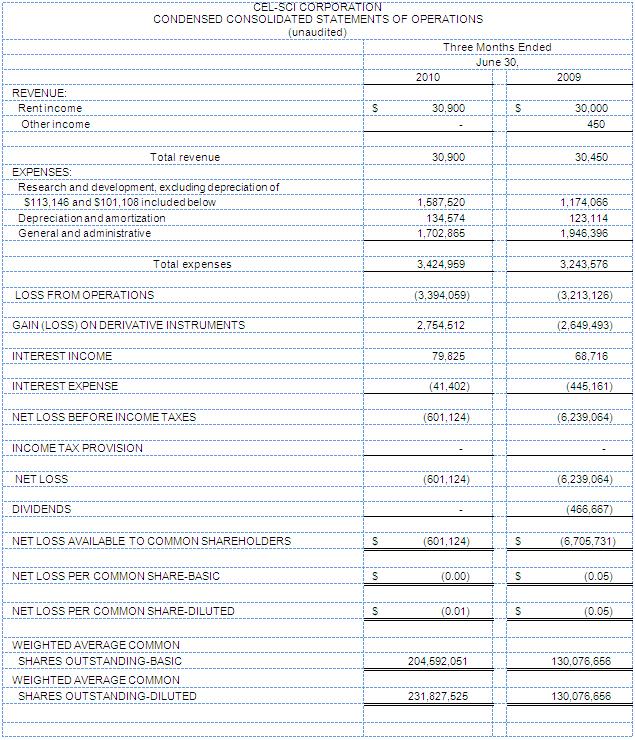
CEL-SCI reported a net loss for the three months ended June 30, 2010 of ($0.6) million versus a loss of ($6.2) million during the same three months in fiscal year 2009. Net loss per basic share was ($0.00) for the three months ended June 30, 2010 versus a loss per basic share of ($0.05) during the same three months in fiscal year 2009. The loss during the quarter ended June 30, 2010 was reduced by a gain on derivative instruments of $2.75 million.
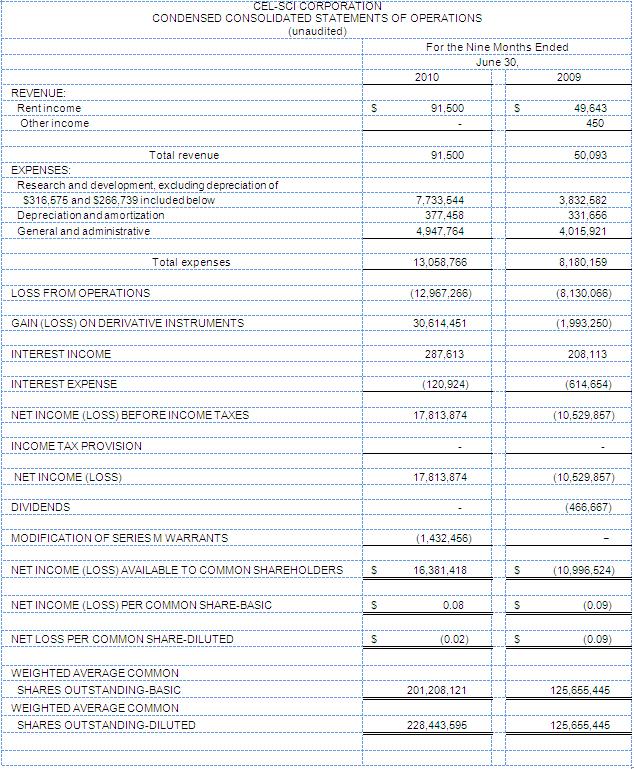
Net income for the nine months ended June 30, 2010 was $17.8 million, versus a loss of ($10.5) million during the same nine months in fiscal year 2009. Net income per basic share was $0.08 for the nine months ended June 30, 2010 versus a net loss per basic share of ($0.09) during the same nine months in fiscal year 2009. The gain on net income for the nine months ended June 30, 2010 was due to derivative accounting.
R&D expenses for the three months ended June 30, 2010 totaled $1.6 million versus R&D expenses of $1.2 million for the same period in fiscal year 2009. R&D expenses for the nine months ended June 30, 2010 totaled $7.7 million versus R&D expenses of $3.8 million for the same period in fiscal year 2009.
As of June 30, 2010, the Company had cash and cash equivalents of $30.5 million. CEL-SCI utilized approximately $3.4 million in operations during the quarter ended June 30, 2010.
Geert Kersten, Chief Executive Officer, said, "We have been diligently preparing to commence the pivotal Phase III trial of our cancer drug MultikineŽ. During the quarter, we invested significantly in personnel, our manufacturing facility and other areas related to supporting the production of the drug, which will be manufactured at our Facility located outside of Baltimore. We are very pleased to have the ability to self-fund our upcoming pivotal study, expected to be the largest head and neck cancer Phase III trial ever conducted, and are working closely with our partners Teva Pharmaceuticals and Orient Europharma towards a successful launch."
About Multikine Cancer Immunotherapy
CEL-SCI is developing Multikine for approval as a first-line indication in newly diagnosed head and neck cancer. To that end, the Company's planned Phase III clinical trial is an 880 patient randomized, controlled clinical study designed to demonstrate that administration of its cancer drug Multikine to head and neck cancer patients before they receive any other conventional cancer treatment will increase their overall survival. In Phase II clinical trials Multikine was shown to be safe and well-tolerated, and to improve the patients' overall survival by 33% at a median of three and a half years following surgery.
The U.S. Food and Drug Administration (FDA) gave the go-ahead for a Phase III clinical trial with Multikine and has granted CEL-SCI's Multikine an Orphan Drug Designation for the "neoadjuvant therapy in patients with squamous cell carcinoma of the head and neck (SCCHN)". In early 2010 CEL-SCI finished the validation of its dedicated state of the art commercial-ready manufacturing facility for Multikine.
Multikine is the first immunotherapeutic agent being developed as a first-line standard of care treatment for cancer. It is administered prior to any other cancer therapy because that is the period when the anti-tumor immune response can still be fully activated. Once the patient has advanced disease, or had surgery or has received radiation and/or chemotherapy, the immune system is severely weakened and is less able to mount an effective anti-tumor immune response. Other immunotherapies are administered after the patient has received chemotherapy and/or radiation therapy, which can limit their effectiveness.
Multikine is a patented defined mixture of naturally derived cytokines. It is the first immunotherapeutic agent in a new class of drugs called "Immune SIMULATORS". Immune SIMULATORS simulate the way our natural immune system acts in defending us against cancer.
About CEL-SCI Corporation
CEL-SCI Corporation is developing products that empower immune defenses. Its lead product Multikine is being readied for a global Phase III trial in advanced primary head and neck cancer. CEL-SCI is also developing an immunotherapy (LEAPS-H1N1-DC) to treat H1N1 hospitalized patients and a vaccine (CEL-2000) for Rheumatoid Arthritis using its L.E.A.P.S. technology platform. The LEAPS-H1N1-DC treatment involves non-changing regions of H1N1 Pandemic Flu, Avian Flu (H5N1), and the Spanish Flu as CEL-SCI scientists are very concerned about the creation of a new more virulent hybrid virus through the combination of H1N1 and Avian Flu, or maybe Spanish Flu. This investigational treatment is currently being tested in a clinical study at Johns Hopkins University. The Company has operations in Vienna, Virginia, and in/near Baltimore, Maryland.
For more information, please visit www.cel-sci.com.
|

